
Biotechnology / CRISPR
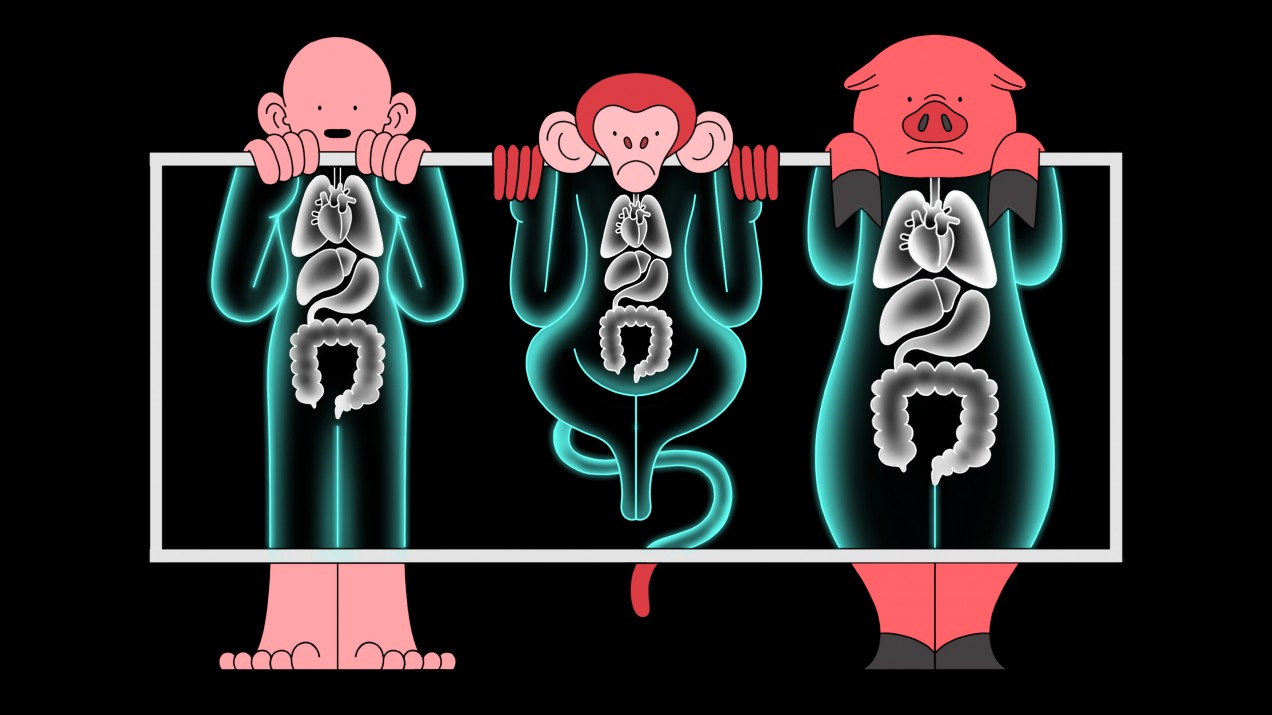
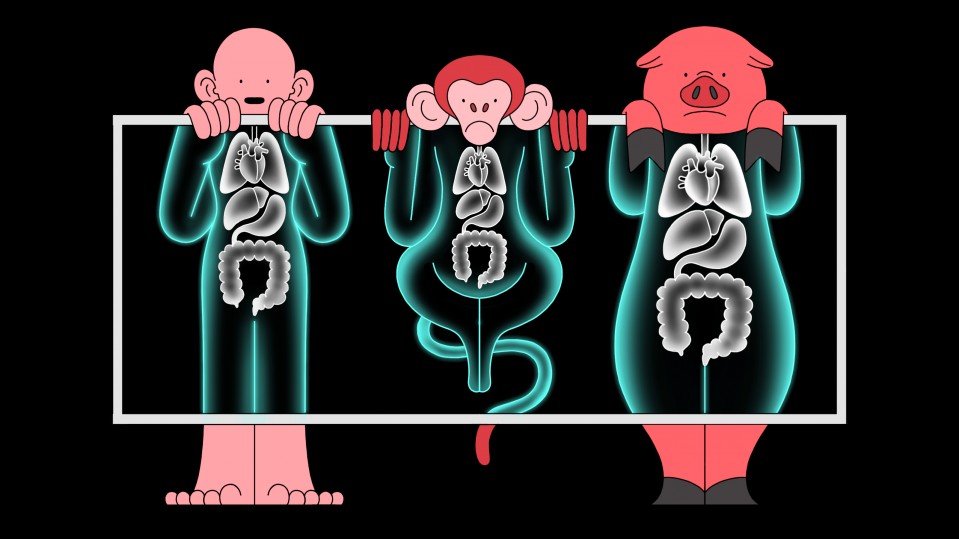
A CRISPR startup is testing pig organs in monkeys to see if they’re safe for us
Gene-editing company eGenesis is now carrying out experiments to help solve a critical shortage of human organs available for transplant.
In 2017, Harvard University geneticist George Church predicted that gene-edited pig organs would be transplanted into people within two years—maybe just one.
“I was wrong,” Church now admits.
A startup he cofounded, eGenesis, had made news for its ambitious plans to use CRISPR gene-editing technology to modify pigs so their organs could be safely transplanted into humans without being rejected. That could solve a critical shortage of human organs available for transplant.
But no human test has yet been carried out. Instead, the company is currently testing organs from its pigs in monkeys at Massachusetts General Hospital in Boston. The experiments are being led by the hospital’s chief of transplant surgery, James Markmann.
“What we’re doing is a necessary step,” says Markmann, also an adviser to the company. “We’d be hard pressed to put a modified organ into a human until it’s been tested in a large animal.”
Neither Markmann nor eGenesis would detail which organs are being studied, or which species of monkey is involved in the experiments, which both say involve the most highly-engineered pig organs that surgeons ever created.
Doctors have dreamed for decades of using pigs to solve the organ shortage by transplanting their kidneys, hearts, and even lungs into human patients to replace organs that have stopped working. Right now, more than 100,000 Americans are waiting on transplant lists.
In the last few years scientists have achieved some major milestones toward such “xenotransplantation.” Researchers at the National Institutes of Health have kept pig hearts beating inside baboons (alongside the monkey’s own heart) for about two years, and German surgeons reported late last year that several baboons had survived for about six months after their hearts were swapped with one from a pig.
Those experiments were carried out using pigs genetically engineered by Revivicor, a subsidiary of United Therapeutics. The animals have genetic changes meant to prevent immediate human rejection of the organ, stop blood clots, and offset other types of immune attacks.
Thanks to such scientific advances, transplant surgeons are now debating how soon a test in a human might be risked. “We’ve got a Chevy. We may even have a BMW now. Do we wait for a Ferrari? There’s a point where you just want to give it a test drive,” says Devin Eckhoff, director of the medical school transplantation division at the University of Alabama at Birmingham.
Before pig organs can be tested in humans, however, there are still some key problems to overcome. Results with monkeys haven’t been very consistent, regulators haven’t said publicly under what conditions they’ll agree to a human test, and there’s debate over just how extensively modified the pigs should be.
Church’s company, eGenesis, leapt to notoriety by touting the role of CRISPR gene editing in swiftly making pigs with multiple genetic modifications—more than had previously been possible. In 2015, cofounder and chief scientist Luhan Yang demonstrated that she could carry out 62 simultaneous edits, used to deactivate viruses that naturally lurk in the pig’s genome.
In addition, Yang says her company has now introduced a “double digit” number of gene edits (both cutting and adding genes) to make the organs less likely to trigger immune rejection. These alterations are probably similar to those created by Revivicor. She has called her pigs the “most advanced” genetically modified animals on earth.
In 2017, speaking to the Carnegie Institution, Church declared that “we hope to do transplants to humans within a year.”
That time line was not realistic. Yang believes a major remaining challenge for the field is to get consistent results in pig-to-monkey transplants. Although sometimes baboons have lived for months with pig organs, other animals die quickly. Researchers don’t yet understand why. “We feel there is some biological reason for that,” Yang says. “We are investigating and trying to fix that.”
Doing so requires large numbers of pig organs. Yang says eGenesis has produced more than 100 pigs in the US, and its Chinese partner Qihan Biotech, based in Hangzhou, has raised hundreds, experimenting with different genetic changes. Regulations don’t allow the pigs, or their organs, to move between countries.
“I think we’ll learn a lot more when we’ve transplanted multiple organs with the same modifications and see how they behave,” says Markmann.
When it comes to the use of animals, likes pigs and baboons, companies are trying to be as discreet as possible. They don't say where the pigs are kept and, in an interview, Markmann would not say the word monkey, instead using the term "large animal." The animal rights organization PETA says it opposes the research because "pigs are individuals, not spare parts."
There also remains wide debate about how many genetic alterations are really necessary. Muhammad Mohiuddin, director of the program in cardiac xenotransplantation for the University of Maryland School of Medicine, believes removing the viral genes is “overkill” and could harm the animals if it leads to unintended effects. Instead, he thinks the sweet spot will be eight or nine gene edits to yield a pig with multiple usable organs—rather than making one pig for heart transplants and another for kidneys. “Otherwise the organs go to waste,” he says.
Markmann says previously published experiments on monkeys and his own recent work make him optimistic that gene-edited pigs will become a viable source of organs for humans. “The fact that there are pig organs surviving for six months or a year, or a couple of years, is really extraordinary, and it says this can be done,” he says. “Everybody sees that we’re at a turning point.”
Correction: A previous version of the article incorrectly described Massachusetts General Hospital surgeon James Markmann as a co-founder of biotech company eGenesis. He is a member of the company's scientific advisory board, not a co-founder.