Oil Spill Sponge
A unique nanotube material may beat the best sorbents on the market


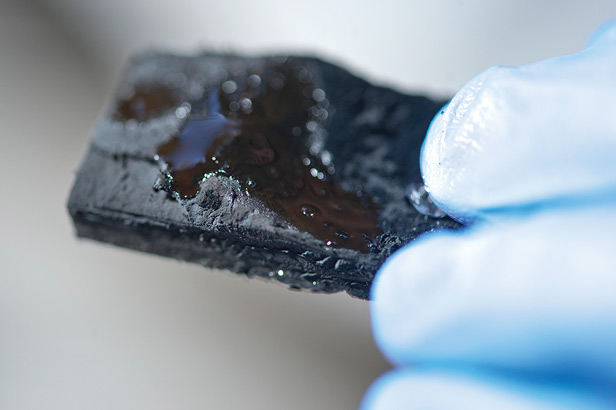
Source: “Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions”
Pulickel M. Ajayan et al.
Nature Scientific Reports, published online April 13, 2012
Results: Adding a small amount of boron to carbon nanotubes helped bond them together to form a strong, three-dimensional network. The result was a resilient, ultralight sponge that could soak up more than 100 times its weight in oil and repel water.
Why it matters: Scientists have had trouble bonding nanotubes together so that they can form large structures, but the new technique provides a way to do that. The first application could be making sorbents for cleaning up oil spills. Initial measurements suggest that the sponge can absorb more than three times as much oil or solvent as the best sorbents on the market but does not take up water, unlike some used now.
The sponge could be reused after squeezing out the oil or burning it off. Among other potentially useful qualities, it has good conductivity, can easily be magnetized, and is one of the lowest-density solids in existence. Its properties might make it useful for other applications, such as high-performance battery electrodes or scaffolding for growing bone tissue.
Methods: Researchers formed nanotubes by creating a mist made of microdroplets of chemical ingredients and then heating it in a furnace. The presence of boron introduced kinks into the nanotubes and helped form strong, covalent bonds between them, which led them to form a three-dimensional structure.
Next Steps: The researchers plan a controlled study to test how the sponge compares with other products on the market in remediating oil spills. In addition, they intend to perform toxicity studies to determine the sponge’s usefulness for biological applications.