Rewriting Life
The Patient of the Future
Internet pioneer Larry Smarr’s quest to quantify everything about his health led him to a startling discovery, an unusual partnership with his doctor, and more control over his life.
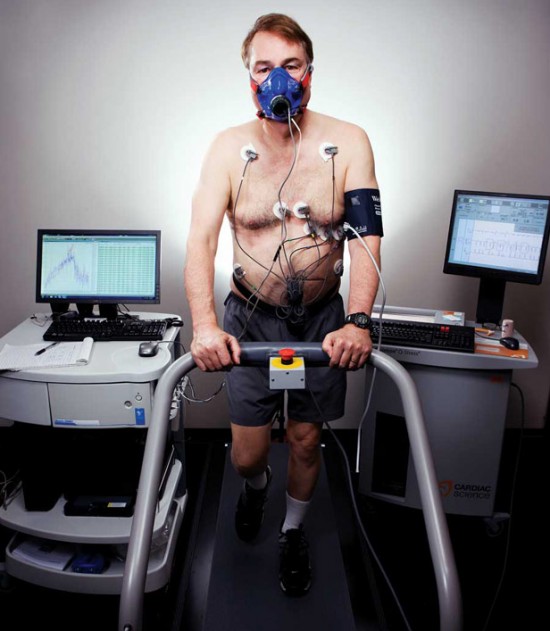

Back in 2000, when Larry Smarr left his job as head of a celebrated supercomputer center in Illinois to start a new institute at the University of California, San Diego, and the University of California, Irvine, he rarely paid attention to his bathroom scale. He regularly drank Coke, added sugar to his coffee, and enjoyed Big Mac Combo Meals with his kids at McDonald’s. Exercise consisted of an occasional hike or a ride on a stationary bike. “In Illinois they said, ‘We know what’s going to happen when you go out to California. You’re going to start eating organic food and get a blonde trainer and get a hot tub,’ ” recalls Smarr, who laughed off the predictions. “Of course, I did all three.”
Smarr, who directs the California Institute for Telecommunications and Information Technology in La Jolla, dropped from 205 to 184 pounds and is now a fit 63-year-old. But his transformation transcends his regular exercise program and carefully managed diet: he has become a poster man for the medical strategy of the future. Over the past decade, he has gathered as much data as he can about his body and then used that information to improve his health. And he has accomplished something that few people at the forefront of the “quantified self” movement have had the opportunity to do: he helped diagnose the emergence of a chronic disease in his body.
Like many “self-quanters,” Smarr wears a Fitbit to count his every step, a Zeo to track his sleep patterns, and a Polar WearLink that lets him regulate his maximum heart rate during exercise. He paid 23andMe to analyze his DNA for disease susceptibility. He regularly uses a service provided by Your Future Health to have blood and stool samples analyzed for biochemicals that most interest him. But a critical skill separates Smarr from the growing pack of digitized patients who show up at the doctor’s office with megabytes of their own biofluctuations: he has an extraordinary ability to fish signal from noise in complex data sets.
On top of his pioneering computer science work—he advocated for the adoption of ARPAnet, an early version of the Internet, and students at his University of Illinois center developed Mosaic, the first widely used browser—Smarr spent 25 years as an astrophysicist focused on relativity theory. That gave him the expertise to chart several of his biomarkers over time and then overlay the longitudinal graphs to monitor everything from the immune status of his gut and blood to the function of his heart and the thickness of his arteries. His meticulously collected and organized data helped doctors discover that he has Crohn’s, an inflammatory bowel disease.
I have ulcerative colitis, a cousin of Crohn’s, and I am intrigued by what Smarr calls his “detective story.” His investigation of his body has evolved into a novel collaboration with a leading gastroenterologist to better understand and treat his disease, and maybe even to help others like me. But I am also a disease-weary skeptic. After 22 years of seeing specialists, enduring a battery of tests, unscrambling the complex medical literature, and trying a hodgepodge of interventions, I have had no luck staving off flares and only modest success controlling them with blunt-force drugs. Like others who have chronic illnesses, I am acutely sensitive to false hope. I have been repeatedly baffled by the course my disease takes and thoroughly confused by tests meant to clarify my condition.
When I first meet Smarr and he gives me a tour of his institute, commonly known as Calit2, I tell him that I find it difficult to separate promise from hype, noting that his endeavor has all the pitfalls of any “n = 1” experiment—a test in which only one person is the subject. “Every disruption begins with an n of 1,” he replies.
Smarr has a standard-issue office on the side of a sleek six-story building, but much of his floor resembles a hip architectural firm. Workstations zigzag across a vast space that features exposed venting pipes and electrical conduits on the naked ceiling. His chief assistant, who lives near San Francisco, talks to coworkers via Skype and a dedicated computer monitor. Across the room, chairs are arranged before a wall of 30-inch displays stacked five high and 14 wide, with a total of 286.7 million pixels that can simultaneously show dozens of brain scans or the stars in a galaxy.
Though he has no laboratory of his own, he shows off the projects at Calit2 as though each were one of his children. The labs investigate everything from machine perception and game culture to integrated nanosensors and 3-D virtual reality. One, which Smarr recently tapped to determine his peak oxygen consumption and maximum heart rate, studies ways to improve individual and population health. Another researches digitally enabled genomic medicine—a blend of self-quantification devices with wireless technology and DNA data.
The place makes my imagination dance. So, too, does Smarr’s medical sleuthing of his own body. Not only does he want to convince others that they can fundamentally alter the patient-doctor relationship and transform physicians into partners, but he’s also going public with his biodata, hoping to crowdsource information that will lead to new insights about the elusive links between DNA sequences, biomarkers, and disease. I soon buy into his vision, embarking on a closer examination of my own disease that, at the very least, scuttles my resignation to it.
MYSTERY SOLVED
Larry Smarr stumbled into his role as a proselytizer for digitizing and then crowdsourcing medicine; he stresses that by nature he is a reserved and private person. He was born and raised in Columbia, Missouri, where his parents ran a flower shop from the home basement. One of his greatest passions is the quiet, solitary cultivation of that most finicky and delicate of plants, the orchid. Yet he has no regrets about going public in writings and talks with extremely intimate details about his body. “Most people think I’m crazy,” he says. But as a result of his candor, many people have contacted him, he says, and he shows me how a Google search on his name now pulls up articles about his quantified-health quest before everything else he has published in his distinguished career.
Smarr says he “got outed as a quantified self” after he spoke at a technology summit in May 2010. A session titled “BioNanoInfo Technology: The Big Challenges” featured him on a panel with Leroy Hood, a cofounder of the Institute for Systems Biology in Seattle and one of the inventors of the first automated DNA sequencer. Hood discussed his push for technology that he hopes will introduce an era of medicine he calls P4: predictive, preventive, personalized, and participatory. Smarr told his own story of using self-quantification to lose weight. A reporter interviewed him after the session, seeking more details, and in the wake of that article, speaking requests started to pour in.
Hood envisions a day when devices using nanotechnology will measure 2,500 markers in blood to track fluctuations in what he estimates are about 50 proteins in 50 of the body’s organs. But that is not yet practical, so Smarr settled on about 100 biomarkers to understand how his dietary changes were affecting his body. Levels of one of the markers, C-reactive protein, or CRP, stood out as higher than normal.
CRP triggers an immune response by binding to the surface of ailing cells, and the level of it should be less than one milligram per liter of blood. Smarr’s level in November 2007 was 6.1. More alarming still, over the next seven months it steadily climbed to 11.8. He felt fine, but he decided to seek a doctor’s advice, worried that something was amiss. The doctor dismissed Smarr’s self-charted longitudinal CRP data, telling him to return if he had symptoms. “Doctors are the gatekeepers, and they’re worried about getting disintermediated,” he says, comparing them to the bank tellers who initially bad-mouthed ATMs.
Within a few months, a sharp, persistent pain in the left side of his abdomen sent him to the doctor’s office, and he was diagnosed with acute diverticulitis, an infection of pockets in the wall of the colon. A blood test showed that his CRP had climbed to 14.5 during the attack. He took antibiotics, the symptoms resolved, and his CRP dropped to 4.9—but that was still unusually high. Concerned that these readings might, as he had read, indicate a plaque buildup that could lead to a heart attack, he had doctors do ultrasounds of his carotid artery and found that it was indeed thickening.
To better understand the attack, he had his stool analyzed for, among other things, lactoferrin, a marker of inflammation. His lactoferrin, too, rose several times to sky-high levels—200, whereas the normal count is less than 7.3. When he overlaid his results on a graph with his CRP fluctuations, he noticed that the two roller-coastered in tandem. A colonoscopy in December 2010 revealed extensive diverticulitis, but Smarr, who had trolled the medical literature online, remained unconvinced that this was his underlying disorder. He became particularly intrigued by studies that linked high lactoferrin levels to inflammatory bowel disease.
At this point, Smarr discovered that UCSD had recently hired a new head of gastroenterology, William Sandborn, who had published a compelling study that charted rises in lactoferrin levels during flares of inflammatory bowel disease. The two met and decided to do yet another colonoscopy. By then, Smarr’s lactoferrin level had risen to a whopping 900. Sandborn reviewed the results and concluded that his new patient might have Crohn’s disease. Smarr now thinks his diverticulitis attack was actually a Crohn’s flare.
“It’s a paradigm for what will happen in the future,” Hood says of Smarr’s story. “With P4 medicine, consumers are going to be the driving force—it isn’t going to be physicians. They’re going to demand to quantize themselves about their own wellness and what can be done.”
Cardiologist Eric Topol, author of The Creative Destruction of Medicine (see “Technological Healing” ) and head of the Scripps Translational Science Institute down the street from UCSD, supports the self-quantification movement but says it has the most to offer people who, like Smarr, zoom in on specific issues. “My colleagues have a doctors-know-best attitude,” says Topol. “Individuals like Larry have much more invested here, and they’re going to put in time and resources to gather as much information as possible. Those clinicians who have the plasticity to adapt to this will be better doctors in the future.”
Smarr recognizes that many people do not have his skills at amassing and analyzing data, nor do they have his resources—he estimates that his “burn rate” for tests and other expenses his health insurance would not pay for has ranged from $5,000 to $10,000 per year. Still, he thinks medical quests like his will become more common with the emergence of technologies that more easily and cheaply test biomarkers and sequence DNA. “My particular story is a good example of an early victory,” he says. “I’m not saying we need to get rid of doctors. But imagine if you go in to the doctor and little widgets have been recording data to the cloud and the doctor can look at it. That’s going to be a vastly more productive visit. There’ll be a liberating effect on them.”
GUT CHECK
Unlike the doctors who deemed Smarr’s data mining a clinically useless “academic” exercise by an amateur, Sandborn welcomes his input. “I’ve learned an enormous amount from listening to patients over the years and just being open-minded about the journey that they go through with their illness,” says the gastroenterologist. Yet Smarr’s unusual project and personality have clearly encouraged Sandborn to explore a patient-doctor relationship of a kind he might have avoided with others. Sandborn notes that in many cases, overtesting wastes money, sends patients on tangents, and can lead to false positive results that actually cause harm. “None of those things apply in Larry’s case,” he says.
Sandborn has agreed to accompany Smarr on an expedition into another medical frontier: the microbiome. In 2010, Nature published a study that sifted through fecal samples from 124 people, plucking out the microbial genes in healthy individuals and those with Crohn’s or ulcerative colitis. In the healthy group, the researchers found an average of 3.3 million microbial genes—about 150 times the number of genes in the human genome. People who had an inflammatory bowel disease harbored 25 percent fewer microbial genes, and the species of bacteria that were depleted differed in people with Crohn’s and those with ulcerative colitis.
Smarr being Smarr, he decided to have his microbiome sequenced at the J. Craig Venter Institute. Sandborn, in turn, plans to work with researchers at the Venter Institute to assess whether they can pull something meaningful out of this most basic data, coupled with Smarr’s biomarkers and the evolution of his disease. Future treatments, for example, might specifically repopulate the gut with the bacteria that people with the disease are lacking. Smarr also plans to have his entire genome sequenced by George Church, the Harvard University geneticist whose Personal Genome Project recruits people willing to share medical records and DNA sequences. “Larry and a few others are becoming very well-measured individuals,” says Church. “What we’re trying to do is gather together such individuals and turn it into more of a collective process. If you keep data to yourself, it’s hard to interpret.”
Larry Smarr has not convinced me that I can manage my ulcerative colitis more effectively by following his lead. But his experience has prodded me to consider options I previously discounted or didn’t know about. I had 23andMe analyze my single-nucleotide polymorphisms, which spotlighted a mutant immune-system gene I carry that almost doubles my risk for ulcerative colitis. I joined the Personal Genome Project—which will also sequence my microbiome—and agreed to make all my DNA and medical records public. I saw Sandborn as a patient, and we plan to monitor my CRP and lactoferrin during a flare and on medication. If I can find immune-modulating drugs on the market that specifically counteract the effects of my mutant gene and do not have serious side effects, Sandborn says, he’s willing to try those on me too.
At the end of my consultation with Sandborn, it becomes clear that we share a sense of skepticism and hope about the new medical world that Larry Smarr has encouraged each of us to enter. “I have no doubt this is the future of medicine, but I have no idea how to get there from here,” he says. “Then again, when you find the right patients, you can start to figure out how to move forward.”
TR contributing editor Jon Cohen is a correspondent with Science. His latest book is Almost Chimpanzee: Redrawing the Lines that Separate Us From Them.